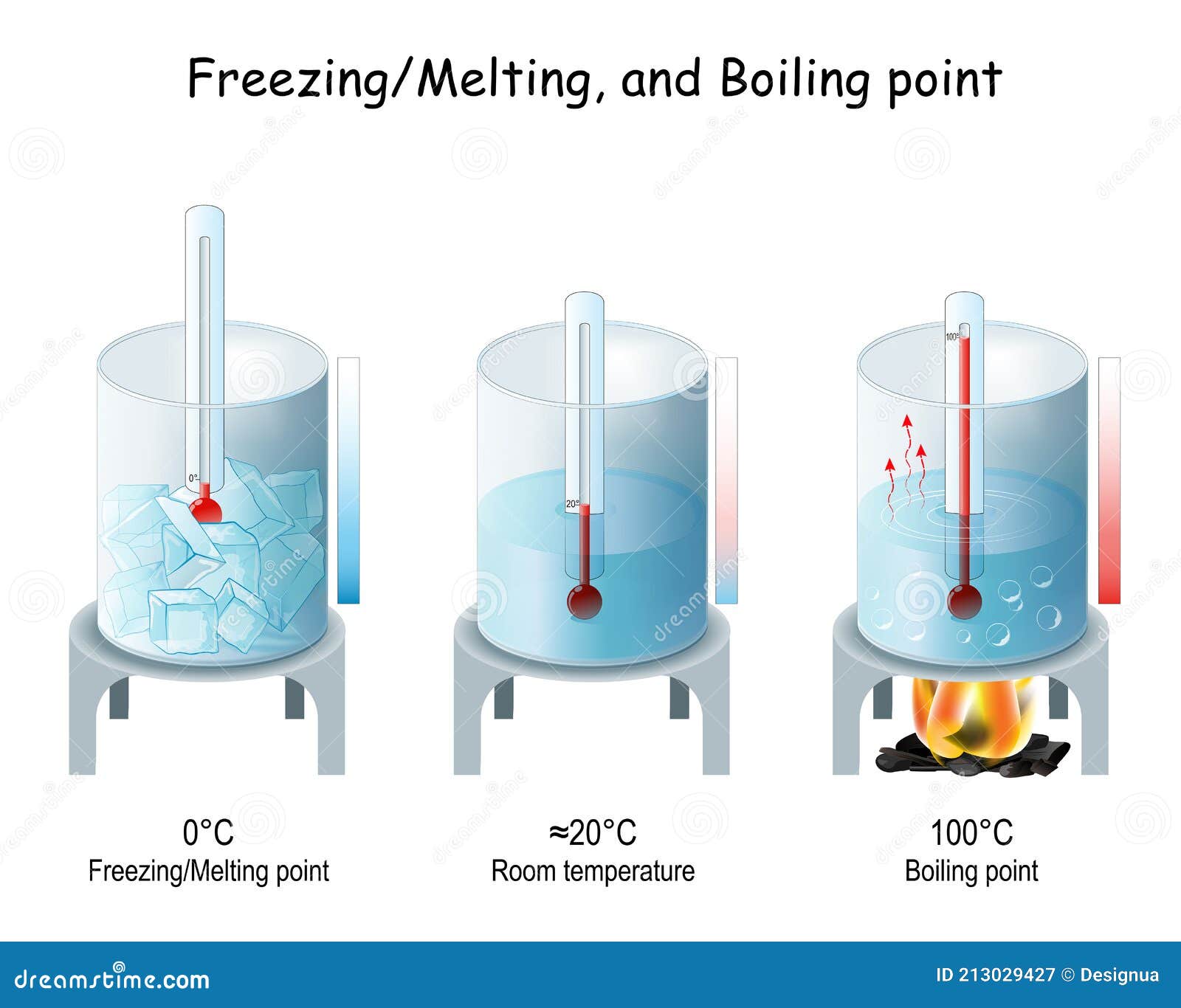
Sparkling Water Freezing Point . the liquid is chilled to below its nominal freezing point, but it doesn't transition to solid, largely because there isn't any. Why does sparkling water have a lower freezing point than flat water? yes, you can freeze carbonated water! pure water freezes at 32°f (0°c), but dissolved salts and minerals can lower the freezing point. carbonated water still freezes at the standard freezing temperature because the majority of the drink is still water and there is not enough carbon dioxide in the water to truly affect the amount of time it will take to freeze. Carbonation lowers the freezing point. However, it is crucial to handle it with caution and follow specific guidelines to. Open the bottle or can of. how to freeze sparkling water safely? To freeze sparkling water safely, follow these steps:
from www.dreamstime.com
pure water freezes at 32°f (0°c), but dissolved salts and minerals can lower the freezing point. To freeze sparkling water safely, follow these steps: However, it is crucial to handle it with caution and follow specific guidelines to. Why does sparkling water have a lower freezing point than flat water? Carbonation lowers the freezing point. Open the bottle or can of. how to freeze sparkling water safely? the liquid is chilled to below its nominal freezing point, but it doesn't transition to solid, largely because there isn't any. yes, you can freeze carbonated water! carbonated water still freezes at the standard freezing temperature because the majority of the drink is still water and there is not enough carbon dioxide in the water to truly affect the amount of time it will take to freeze.
Boiling and Evaporation, Freezing and Melting Points of Water Stock
Sparkling Water Freezing Point the liquid is chilled to below its nominal freezing point, but it doesn't transition to solid, largely because there isn't any. Why does sparkling water have a lower freezing point than flat water? yes, you can freeze carbonated water! pure water freezes at 32°f (0°c), but dissolved salts and minerals can lower the freezing point. To freeze sparkling water safely, follow these steps: However, it is crucial to handle it with caution and follow specific guidelines to. Carbonation lowers the freezing point. Open the bottle or can of. carbonated water still freezes at the standard freezing temperature because the majority of the drink is still water and there is not enough carbon dioxide in the water to truly affect the amount of time it will take to freeze. the liquid is chilled to below its nominal freezing point, but it doesn't transition to solid, largely because there isn't any. how to freeze sparkling water safely?
From www.bartleby.com
Freezing Point of Water bartleby Sparkling Water Freezing Point pure water freezes at 32°f (0°c), but dissolved salts and minerals can lower the freezing point. how to freeze sparkling water safely? However, it is crucial to handle it with caution and follow specific guidelines to. To freeze sparkling water safely, follow these steps: Why does sparkling water have a lower freezing point than flat water? the. Sparkling Water Freezing Point.
From ksa.mytutorsource.com
Water Freezing Point Definition, Factors Affecting It & Supercooled Sparkling Water Freezing Point yes, you can freeze carbonated water! However, it is crucial to handle it with caution and follow specific guidelines to. Carbonation lowers the freezing point. To freeze sparkling water safely, follow these steps: how to freeze sparkling water safely? Open the bottle or can of. pure water freezes at 32°f (0°c), but dissolved salts and minerals can. Sparkling Water Freezing Point.
From studyfullwexler.z1.web.core.windows.net
Salt Water Freezing Point Chart Sparkling Water Freezing Point However, it is crucial to handle it with caution and follow specific guidelines to. pure water freezes at 32°f (0°c), but dissolved salts and minerals can lower the freezing point. To freeze sparkling water safely, follow these steps: Carbonation lowers the freezing point. carbonated water still freezes at the standard freezing temperature because the majority of the drink. Sparkling Water Freezing Point.
From www.numerade.com
SOLVED Constants for freezingpoint depression and boilingpoint Sparkling Water Freezing Point Why does sparkling water have a lower freezing point than flat water? However, it is crucial to handle it with caution and follow specific guidelines to. yes, you can freeze carbonated water! carbonated water still freezes at the standard freezing temperature because the majority of the drink is still water and there is not enough carbon dioxide in. Sparkling Water Freezing Point.
From www.pinterest.jp
Water Freezing Point Definition, Factors Affecting It & Supercooled Sparkling Water Freezing Point carbonated water still freezes at the standard freezing temperature because the majority of the drink is still water and there is not enough carbon dioxide in the water to truly affect the amount of time it will take to freeze. pure water freezes at 32°f (0°c), but dissolved salts and minerals can lower the freezing point. the. Sparkling Water Freezing Point.
From betterlesson.com
Lesson Boiling and Freezing Points Lesson 1 All Hail the Freezing Point! Sparkling Water Freezing Point pure water freezes at 32°f (0°c), but dissolved salts and minerals can lower the freezing point. However, it is crucial to handle it with caution and follow specific guidelines to. Carbonation lowers the freezing point. the liquid is chilled to below its nominal freezing point, but it doesn't transition to solid, largely because there isn't any. yes,. Sparkling Water Freezing Point.
From www.slideserve.com
PPT Salt Changes the Freezing Point of Water PowerPoint Presentation Sparkling Water Freezing Point Carbonation lowers the freezing point. pure water freezes at 32°f (0°c), but dissolved salts and minerals can lower the freezing point. Open the bottle or can of. To freeze sparkling water safely, follow these steps: However, it is crucial to handle it with caution and follow specific guidelines to. how to freeze sparkling water safely? the liquid. Sparkling Water Freezing Point.
From boatbasincafe.com
How Long Does It Take For Ice To Freeze And How To Speed It Up Sparkling Water Freezing Point Why does sparkling water have a lower freezing point than flat water? yes, you can freeze carbonated water! To freeze sparkling water safely, follow these steps: pure water freezes at 32°f (0°c), but dissolved salts and minerals can lower the freezing point. Open the bottle or can of. Carbonation lowers the freezing point. However, it is crucial to. Sparkling Water Freezing Point.
From haydenowen.z21.web.core.windows.net
Water Freezing Point Pressure Chart Sparkling Water Freezing Point yes, you can freeze carbonated water! Why does sparkling water have a lower freezing point than flat water? To freeze sparkling water safely, follow these steps: carbonated water still freezes at the standard freezing temperature because the majority of the drink is still water and there is not enough carbon dioxide in the water to truly affect the. Sparkling Water Freezing Point.
From www.worldatlas.com
What is the Freezing Point in Fahrenheit? WorldAtlas Sparkling Water Freezing Point Why does sparkling water have a lower freezing point than flat water? carbonated water still freezes at the standard freezing temperature because the majority of the drink is still water and there is not enough carbon dioxide in the water to truly affect the amount of time it will take to freeze. yes, you can freeze carbonated water!. Sparkling Water Freezing Point.
From www.metoffice.gov.uk
Sea ice an overview Met Office Sparkling Water Freezing Point Why does sparkling water have a lower freezing point than flat water? Carbonation lowers the freezing point. pure water freezes at 32°f (0°c), but dissolved salts and minerals can lower the freezing point. However, it is crucial to handle it with caution and follow specific guidelines to. the liquid is chilled to below its nominal freezing point, but. Sparkling Water Freezing Point.
From www.thoughtco.com
What Is the Freezing Point of Water? Sparkling Water Freezing Point how to freeze sparkling water safely? carbonated water still freezes at the standard freezing temperature because the majority of the drink is still water and there is not enough carbon dioxide in the water to truly affect the amount of time it will take to freeze. To freeze sparkling water safely, follow these steps: yes, you can. Sparkling Water Freezing Point.
From mavink.com
Ethanol Water Freezing Point Chart Sparkling Water Freezing Point Why does sparkling water have a lower freezing point than flat water? Carbonation lowers the freezing point. yes, you can freeze carbonated water! the liquid is chilled to below its nominal freezing point, but it doesn't transition to solid, largely because there isn't any. To freeze sparkling water safely, follow these steps: carbonated water still freezes at. Sparkling Water Freezing Point.
From www.vrogue.co
Water Freezing Point Pressure Chart vrogue.co Sparkling Water Freezing Point To freeze sparkling water safely, follow these steps: how to freeze sparkling water safely? Open the bottle or can of. carbonated water still freezes at the standard freezing temperature because the majority of the drink is still water and there is not enough carbon dioxide in the water to truly affect the amount of time it will take. Sparkling Water Freezing Point.
From www.researchgate.net
FREEZING POINT DATA FOR AQUEOUS SOLUTIONS OF ETHYLENE GLYCOL (MEG Sparkling Water Freezing Point To freeze sparkling water safely, follow these steps: pure water freezes at 32°f (0°c), but dissolved salts and minerals can lower the freezing point. Open the bottle or can of. Why does sparkling water have a lower freezing point than flat water? yes, you can freeze carbonated water! Carbonation lowers the freezing point. However, it is crucial to. Sparkling Water Freezing Point.
From www.thoughtco.com
What Is the Freezing Point of Water? Sparkling Water Freezing Point To freeze sparkling water safely, follow these steps: how to freeze sparkling water safely? Open the bottle or can of. Carbonation lowers the freezing point. However, it is crucial to handle it with caution and follow specific guidelines to. pure water freezes at 32°f (0°c), but dissolved salts and minerals can lower the freezing point. the liquid. Sparkling Water Freezing Point.
From www.youtube.com
Seltzer Water Freezing Point Elevation demo YouTube Sparkling Water Freezing Point the liquid is chilled to below its nominal freezing point, but it doesn't transition to solid, largely because there isn't any. Open the bottle or can of. how to freeze sparkling water safely? However, it is crucial to handle it with caution and follow specific guidelines to. pure water freezes at 32°f (0°c), but dissolved salts and. Sparkling Water Freezing Point.
From stock.adobe.com
Boiling and Evaporation, Freezing and Melting Points of Water. Stock Sparkling Water Freezing Point pure water freezes at 32°f (0°c), but dissolved salts and minerals can lower the freezing point. the liquid is chilled to below its nominal freezing point, but it doesn't transition to solid, largely because there isn't any. To freeze sparkling water safely, follow these steps: how to freeze sparkling water safely? yes, you can freeze carbonated. Sparkling Water Freezing Point.